Air Stripper Calculator

Use this calculator to select an air stripper model. It is simple; just follow these steps:
- Enter required fields
- Hit RUN button for results
- Need a contaminant modeled which is not on the list? Email your request to TreatmentGroup [at] QEDEnv [dot] com
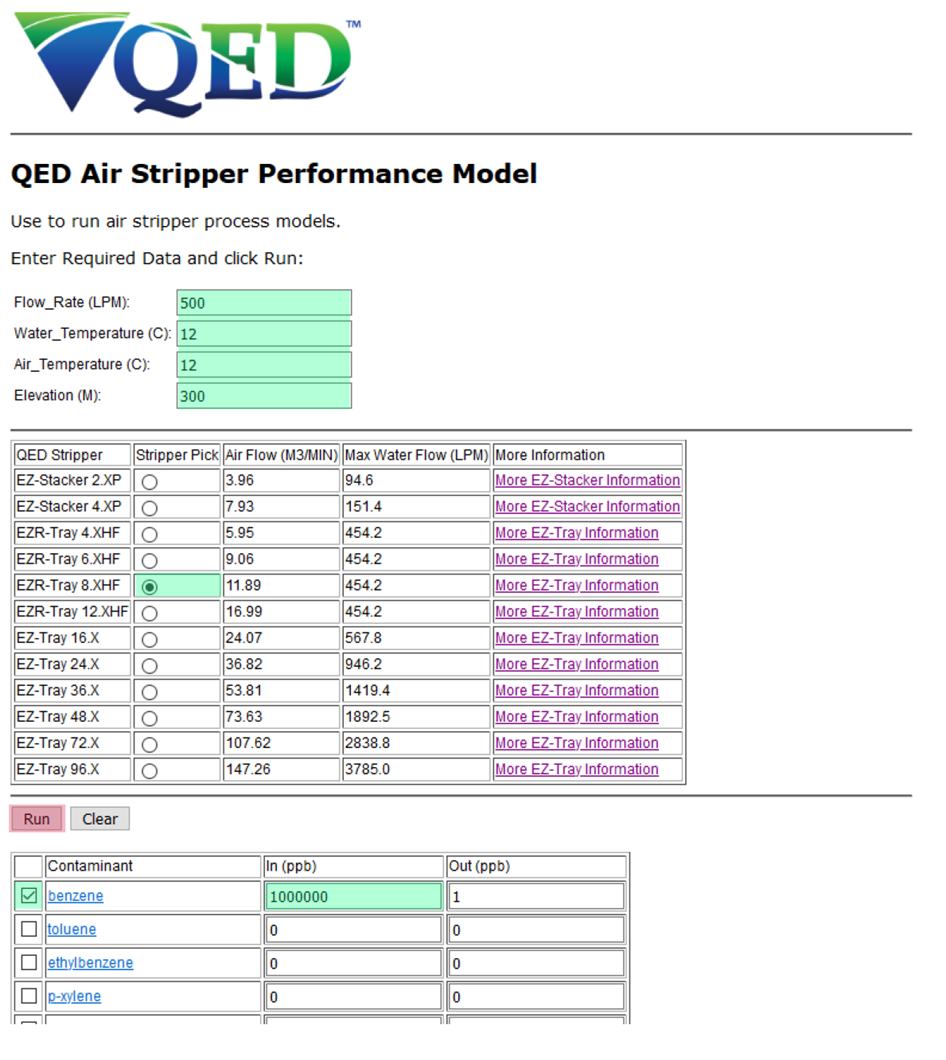 Air Stripper Calculator (Imperial Units)
Air Stripper Calculator (Metric Units)
Air Stripper Calculator (Imperial Units)
Air Stripper Calculator (Metric Units)
